Counterfeit Respirators / Misrepresentation of NIOSH-Approval
Notice on NIOSH ownership of respirator certification marks
NIOSH has successfully recorded the NIOSH stylized logo with and without text, as well as the certification marks N95, N99, N100, P95, P100, and the term “NIOSH-approved”, with the U.S. Patent and Trademark Office (USPTO). NIOSH, as the certifying federal entity for the N95 Respirator Approval Program, owns these certification marks, meaning that NIOSH controls who can use these marks. Accordingly, NIOSH will let manufacturers use these certification marks only if they become NIOSH-approval holders because of their products satisfying the NIOSH’s regulatory standards set forth in 42 C.F.R. Part 84. While these marks have historically been protected under common law (as opposed to a trademark registration) since they were established by the program regulations, these marks are now registered with the USPTO as federal registrations, as well as in various foreign countries, and are subject to additional protections under the Lanham Act, 15 U.S.C. §§ 1051 et seq. and foreign trademark laws. Thus, any misuse of these marks, including on respirators that have failed to satisfy NIOSH’s regulatory requirements or have not received a NIOSH approval, is a direct violation of applicable trademark laws and NIOSH may pursue action as necessary. This also applies to approval holders that misuse or misplace the marks or terms against the regulations, specifically outlined in 42 C.F.R. Part 84.33.
Counterfeit respirators are products that are falsely marketed and sold as being NIOSH-approved and may not be capable of providing appropriate respiratory protection to workers. When NIOSH becomes aware of counterfeit respirators or those misrepresenting NIOSH approval on the market, we will post them here to alert users, purchasers, and manufacturers.
How to identify a NIOSH-approved respirator:
NIOSH-approved respirators have an approval label on or within the packaging of the respirator (i.e. on the box itself and/or within the users’ instructions). Additionally, an abbreviated approval is on the FFR itself. You can verify the approval number on the NIOSH Certified Equipment List (CEL) or the NIOSH Trusted-Source page to determine if the respirator has been approved by NIOSH. NIOSH-approved FFRs will always have one the following designations: N95, N99, N100, R95, R99, R100, P95, P99, P100.
Signs that a respirator may be counterfeit:
- No markings at all on the filtering facepiece respirator
- No approval (TC) number on filtering facepiece respirator or headband
- No NIOSH markings
- NIOSH spelled incorrectly
- Presence of decorative fabric or other decorative add-ons (e.g., sequins)
- Claims for the of approval for children (NIOSH does not approve any type of respiratory protection for children)
- Filtering facepiece respirator has ear loops instead of headbands
Check the respirator approval markings using the Example of Correct Exterior Markings on a NIOSH-Approved Filtering Facepiece Respirator graphic.
More Tips to Spot Counterfeit Respirators
Note – Below the most recent listings are additional counterfeit respirators.
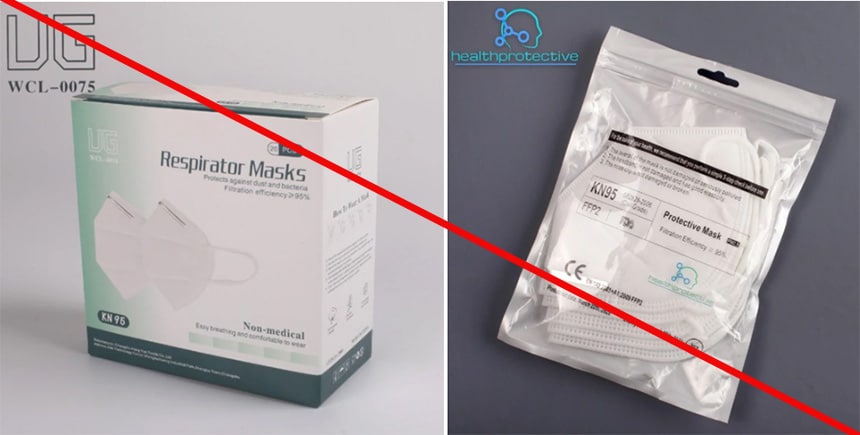
The Health Protective KN95 mask is being marketed as “Certified KN95 respirator mask, adopted by the CDC”. This statement is misleading because CDC, through NIOSH, does not approve KN95 masks or any other respiratory protective device certified to international standards. Additionally, Health Protective is misusing NIOSH test information. The product package indicates it meets Chinese standard GB 2626-2006 and was submitted to NIOSH under an International Respirator Assessment request. It is being marketed using results from the assessment. As stated on the NIOSH website, these results are not to be used by manufacturers, distributors, suppliers, and importers to make claims about their products and/or to influence purchasers and cannot be used to make claims that the product meets NIOSH approval requirements. Changshu City Hengyun Nonwoven Products Co., Ltd. is not a NIOSH approval holder or a private label holder. (1/11/2022)

Guangzhou Zhen Tao Culture Media Co., Ltd. is marketing Benehal Model 8865 as a NIOSH approved unit for kids. NIOSH does not approve filtering facepiece respirators for children. Although Suzhou Sanical Protective Product Manufacturing Co. Ltd. manufactures Benehal Model 8865, under NIOSH approval number TC-84A-7449, this unit is not NIOSH approved as a filtering facepiece respirator for children. (12/17/2021)

This is an example of a misrepresentation of NIOSH approval. SafeShield’s marketing of model FS-N95 is misleading and may cause users to believe it is NIOSH approved. SafeShield references certification to NIOSH CFR 42.84 180-181 and TEB-APR-STP-0059. This information is inaccurate. SafeShield is not a NIOSH approval holder or private label assignee, and model FS-N95 is NOT NIOSH approved. (12/1/2021)

This is an example of a misrepresentation of a NIOSH approval. Megha International is marketing the Feel Safe Mask N95 in a package marked NIOSH Certification. Megha International is not a NIOSH approval holder or private label assignee. Feel Safe Mask N95 is NOT NIOSH approved. (11/5/2021)

Dongguan AOXING is misusing NIOSH test information for its KN95 Protective Mask model AX-KF95. Product was submitted to NIOSH under an International Respirator Assessment request and it is being marketed using results from the assessment. As stated on the NIOSH website, these results are not to be used by manufacturers, distributors, suppliers, and importers to make claims about their products and/or to influence purchasers and cannot be used to make claims that the product meets NIOSH approval requirements. Dongguan AOXING is not a NIOSH approval holder or a private label assignee. (11/5/2021)

This is an example of a misrepresentation of a NIOSH approval. AP Mascarillas is not a NIOSH approval holder or private label assignee. AP Mascarillas is marketing product using the NIOSH logo, but their respiratory protective devices are NOT NIOSH approved. (9/9/2021)

This is an example of a misrepresentation of a NIOSH approval. Sobmex is marketing numerous filtering facepiece respirators with NIOSH listed on the technical specifications sheet, but Sobmex is not a NIOSH approval holder or private label assignee. Sobmex respirators are NOT NIOSH approved. (9/9/2021)

Moaron is NOT a NIOSH approval holder and they are misrepresenting product as meeting NIOSH approval. The product listing claims the filter “meets NIOSH P100-series.” NIOSH only approves whole respirator configurations, not individual components. The Moaron 2091 P100 filter is NOT a component associated with a NIOSH approval. Additionally, it is incorrectly being advertised that it is compatible with other NIOSH-approved products. If this filter is used in place of the filter component associated with the NIOSH-approved respiratory protective device (RPD), it will void the NIOSH approval. (9/9/2021)

This is an example of a misrepresentation of a NIOSH approval. Pure&Safe is not a NIOSH approval holder or a private label assignee. The Pure&Safe 5 Layered Reusable Anti-Pollution N95 Face Mask with Activated Carbon Filter is not NIOSH approved. (5/26/2021)

This is an example of a misrepresentation of a NIOSH approval. U-SAFE is not a NIOSH approval holder or a private label assignee. U-SAFE models B120 and B130 N95 particulate respirator are not NIOSH approved. (4/30/2021)

This is an example of a misrepresentation of a NIOSH approval. Osprey is not a NIOSH approval holder or a private label assignee. The Osprey N95 particulate respirator is not NIOSH approved. (4/21/2021)

EPC Product, LLC is misrepresenting product manufactured by their company as NIOSH-approved product. Models sold by EPC, including but not limited to, PT-N95F-01, PT-N95C-02, and PT-N95CS-02 are NOT NIOSH-approved. EPC Product, LLC is not a NIOSH approval holder or a private label holder. (3/26/2021)

SS Paper Convertors is misrepresenting protective masks as NIOSH-approved. SS Paper Convertors is not a NIOSH approval holder or private label holder. La’ Forte brand masks are not NIOSH-approved. (2/26/2021)

Zhengzhou Ruipu Medical Technology Co., Ltd. is misusing NIOSH test information regarding RUIPU RIPE DOCTORS KN95. The product package indicates it meets Chinese standard GB 2626-2006 and was submitted to NIOSH under an International Respirator Assessment request. It is being marketed using results from the assessment. As stated on the NIOSH website, these results are not to be used by manufacturers, distributors, suppliers, and importers to make claims about their products and/or to influence purchasers and cannot be used to make claims that the product meets NIOSH approval requirements. Zhengzhou Ruipu Medical Technology Co., Ltd. is not a NIOSH approval holder or a private label holder. (2/18/2021)

Chengde Technology Co., Ltd. is misusing NIOSH test information regarding WWDOLL model CD9501B KN95 Foldable Protective Masks. The product package indicates it meets Chinese standard GB 2626-2019 and was submitted to NIOSH under an International Respirator Assessment request. It is being marketed using results from the assessment. As stated on the NIOSH website, these results are not to be used by manufacturers, distributors, suppliers, and importers to make claims about their products and/or to influence purchasers and cannot be used to make claims that the product meets NIOSH approval requirements. Chengde Technology Co., Ltd. is not a NIOSH approval holder or a private label holder. (2/18/2021)
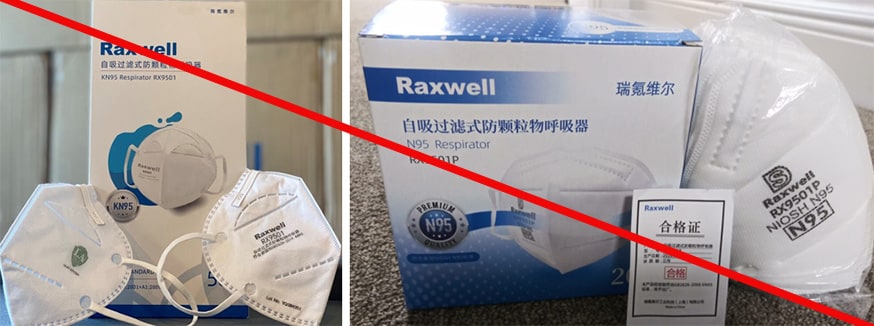
Raxwell Industrial Technology Co., Ltd. is misusing NIOSH test information regarding Raxwell model RX9501 KN95 Face Masks. The product package indicates it meets Chinese standard GB 2626-2006 and was submitted to NIOSH under an International Respirator Assessment request. It is being marketed using results from the assessment. As stated on the NIOSH website, these results are not to be used by manufacturers, distributors, suppliers, and importers to make claims about their products and/or to influence purchasers and cannot be used to make claims that the product meets NIOSH approval requirements. Additionally, Raxwell model RX9501P N95 is being misrepresented as a NIOSH-approved product. Raxwell Industrial Technology Co., Ltd. is not a NIOSH approval holder or a private label holder. (2/18/2021)

This is an example of a misrepresentation of a NIOSH-approved product. Products labeled TENAMYD FM and sold by Clean Life 360 are NOT NIOSH approved. (2/4/2021)
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.